Each unit of heat can be referred to in calories. The amount of energy (heat) required to raise the temp of one Kg of water by one degree C at 1 bar of atmospheric pressure.
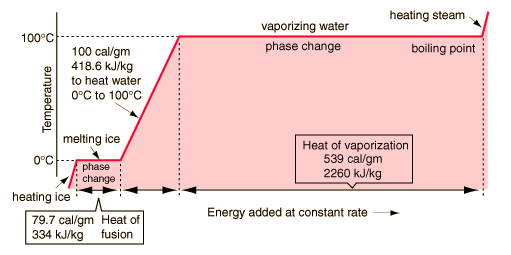
The latent heat of vaporization for water is 539 calories per gram. This is an endothermic reaction, meaning it absorbs heat. Water can reach up to its boiling point but not any higher. The process of vaporization (boiling) is a cooling process. In the process of cooling, the water boils as the vaporized water absorbs energy during the change of phase. Higher pressure means more energy is required to make this change happen.
So we know that vaporization of water absorbs a lot of heat. This is more than any other easily available liquid. Here are some comparisons:
Water 539 cal for enthalpy of vaporization
Ethanol about 201 cal for enthalpy of vaporization
Methanol 264 cal for enthalpy of vaporization
So you can see that the Water absorbs 2x the heat energy from the surrounding air/surfaces during this change of phase. This gives water extremely high anti-detonation properties when injected into the internal combustion engine because it is able to absorb a lot of heat. Under higher pressures, the energy required is even higher. In a typical forced induction application, the water can absorb more than 2x as much energy as water under atmospheric pressure.
A calorie: The energy needed to increase the temperature of a gram of water by 1 °C"
"Water is also commonly expressed as 539.423 calories per gram.
So the amount of energy absorbed by one gram of water during a change of phase is the same amount that it takes to raise the temperature of that same amount 539.4 °C. Of course, water will vaporize at 100°C but that just goes to show... The amount of energy absorbed is extremely high.

The latent heat of vaporization for water is 539 calories per gram. This is an endothermic reaction, meaning it absorbs heat. Water can reach up to its boiling point but not any higher. The process of vaporization (boiling) is a cooling process. In the process of cooling, the water boils as the vaporized water absorbs energy during the change of phase. Higher pressure means more energy is required to make this change happen.
So we know that vaporization of water absorbs a lot of heat. This is more than any other easily available liquid. Here are some comparisons:
Water 539 cal for enthalpy of vaporization
Ethanol about 201 cal for enthalpy of vaporization
Methanol 264 cal for enthalpy of vaporization
So you can see that the Water absorbs 2x the heat energy from the surrounding air/surfaces during this change of phase. This gives water extremely high anti-detonation properties when injected into the internal combustion engine because it is able to absorb a lot of heat. Under higher pressures, the energy required is even higher. In a typical forced induction application, the water can absorb more than 2x as much energy as water under atmospheric pressure.
A calorie: The energy needed to increase the temperature of a gram of water by 1 °C"
"Water is also commonly expressed as 539.423 calories per gram.
So the amount of energy absorbed by one gram of water during a change of phase is the same amount that it takes to raise the temperature of that same amount 539.4 °C. Of course, water will vaporize at 100°C but that just goes to show... The amount of energy absorbed is extremely high.








 Be the first to like this post.
Be the first to like this post.

 Back to top
Back to top
